
Industrial-scale microbial production environments are profoundly different from the cultivation environments commonly used at lab scale. Microbial metabolic pathway discovery and engineering efforts have led to an increasing number of biotechnological processes in diverse sectors of our economy, ranging from energy to health and medicine, as well as food and agriculture. Our approach can be broadly applied to investigate diversion of flux from central carbon metabolism for NRPS and other heterologous pathway engineering, or to follow a population switch between respiratory and non-respiratory modes.
The final product indigoidine is linked to the activity of the TCA cycle and serves as a reporter for the respiratory state of S. This study represents the first use of the Streptomyces lavendulae NRPS (BpsA) in a fungal host and its scale-up. By promoting respiratory conditions via controlled feeding, we scaled the production to a 2 L bioreactor scale, reaching a maximum titer of 980 mg/L.

Production of indigoidine is abolished during non-respiratory growth even under aerobic conditions. We characterize carbon source use and production dynamics, and demonstrate that indigoidine is solely produced during respiratory cell growth. Saccharomyces cerevisiae was engineered to express a bacterial NRPS that converts glutamine to indigoidine. Indigoidine, a blue pigment, is a representative NRP that is valuable by itself as a renewably produced pigment. Non-ribosomal peptide synthetases (NRPS) are an important class of biocatalysts that provide access to a wide array of secondary metabolites. This transition between respiratory and non-respiratory metabolic state is accompanied by substantial modifications of central carbon metabolism, which impact the efficiency of metabolic pathways and the corresponding final product titers.
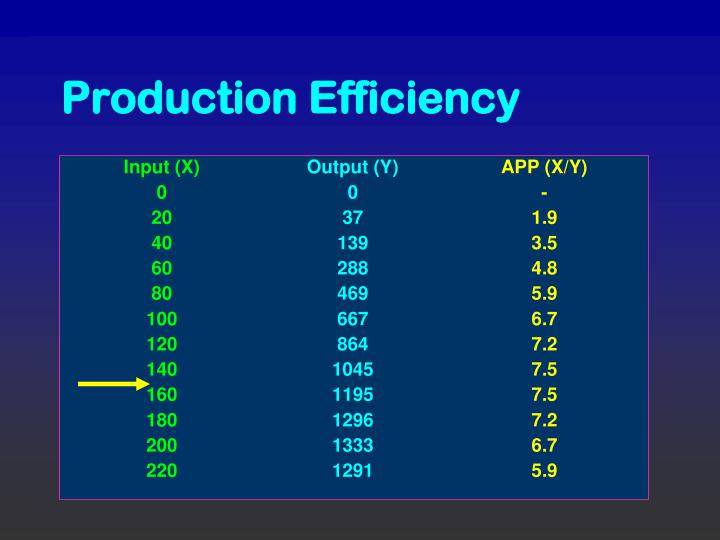
The biotechnologically important yeast Saccharomyces cerevisiae adjusts its energy metabolism based on the availability of oxygen and carbon sources. Beyond pathway engineering, the metabolic state of the production host is critical in maintaining the efficiency of cellular production.


 0 kommentar(er)
0 kommentar(er)
